At absolute zero the value of the chemical potential, μ, is defined as the Fermi energy At room temperature the chemical potential for metals is virtually the same as the Fermi energy – typically the difference is only of the order of 001% Not surprisingly, the chemical potential for metals at room temperature is often taken to be theChemical bond energy is an important example of potential energy Kinetic and potential energy can be interconverted Measuring energy the calorie and kilocalorie (kcal)1 Burning of a candle or most flamecharacterised combustions 2 There are many organisms that give off light Firefly, angelfish, many underwater tubes and worms etc 3 Radium used to be painted on watches to give off light 4 Phosphorus(Whit
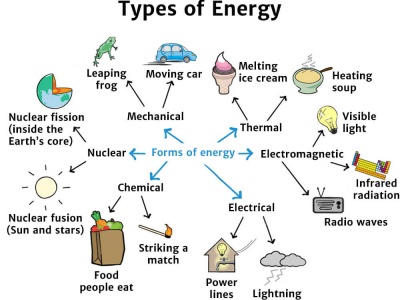
Types Of Energy Knowledge Bank Solar Schools
What is an example of a chemical energy
What is an example of a chemical energy-Chemical energy is stored in the bonds of atoms and molecules – it is the energy that holds these particles together Stored chemical energy is found in food, biomass, petroleum, and natural gas Let's go!Chemical energy is the potential of a chemical substance to undergo a chemical reaction to transform into other substances Some examples of storage media of chemical energy include batteries, food, and gasoline Breaking or making of chemical bonds involves energy, which may be either absorbed or evolved from a chemical system




What Is Chemical Energy Definition And Examples
Start studying Energy Y10a Given the name of the machine state the original form of energy and the desired output form of energy (Eg Given CAR Answer Chemical Potential, Translational Kinetic) Learn vocabulary, terms, and more with flashcards, games, and other study tools Temporary Emergency Exposure Limits (TEELs) TEELs are guidelines designed to predict the response of members of the general public to different concentrations of a chemical during an emergency response incident Note TEEL values are no longer included specifically in ALOHA, see PACs This page discusses the following topicsThe magnitude of the tetrahedral splitting energy is only 4/9 of the octahedral splitting energy, or Δ t =4/9 Δ 0 CSFE = 04 x n (t 2g) 06 x n (e g) Δ t Where, n (t 2g) and n (eg) are the no of electrons occupying the respective levels
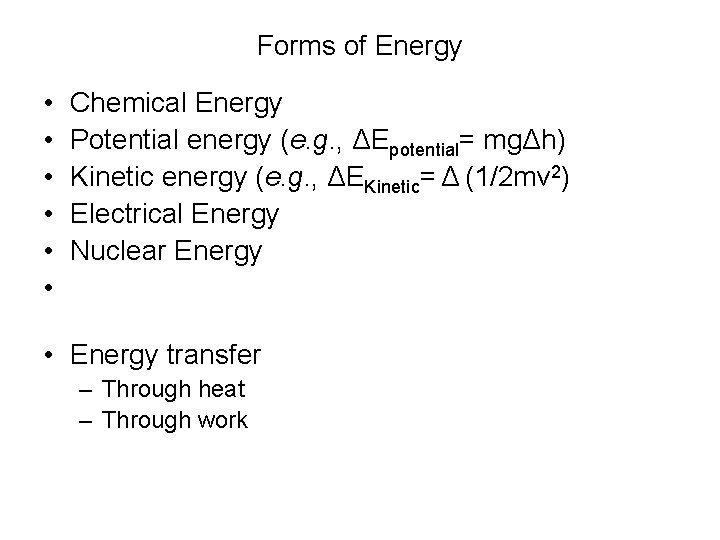
CHEMICAL ENERGY energy stored in chemicals eg fuels, batteries and food It is released by chemical reactions HEAT ENERGY LIGHT ENERGY SOUND ENERGY ELECTRICAL ENERGY Energy is always changing from one form into another The diagram below shows the energy changes inChemical Energy changes Conclusion Energy > Mechanical Home Mechanical energy** There are two main types of mechanical energy They are motion energy and stored mechanical energy Motion energy This is the energy something has because it is moving (eg a speeding cricket ball) You can feel the effect of this energy if the cricket ball Energy transformation is the change of energy from one form to another For example, a ball dropped from a height is an example of a change of energy from potential to kinetic energy Chemical energy from food is converted to mechanical energy when the food is broken down and absorbed in the muscles
They cannot carry out photosynthesis;An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows Reactants → Products Energy What is an Exothermic Reaction?Batteries (eg, leadacid batteries) store chemical energy and convert it to electric energy on demand Batteries do not store electric charge or charge carriers Charge carriers (electrons) enter one terminal of the battery, acquire electrical potential energy, and exit from the other terminal at



Ei Lehigh Edu Learners Energy Readings Energy Basics Pdf
/main-energy-forms-and-examples-609254-v3-5b562a0cc9e77c0037514831.png)



10 Types Of Energy And Examples
The US Department of Energy (DOE) today announced nearly $34 million in funding for 11 projects that will support highimpact research and development to improve and produce biofuels, biopower, and bioproducts Learn more Priorities Combating the Climate Crisis There is no greater challenge facing our nation and our planet than the climateChemical energy is energy stored in the bonds of chemical compounds, like atoms and molecules This energy is released when a chemical reaction takes place Usually, once chemical energy has been released from a substance, that substance is transformed into a completely new substance Solar energydriven sustainable process for synthesis of ethylene glycol from methanol PeerReviewed Publication Dalian Institute of Chemical




What Is Chemical Energy Definition Examples Applications With Videos




Chemical Energy Kids Britannica Kids Homework Help
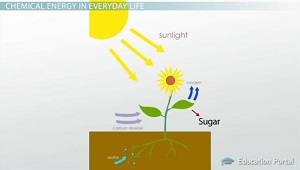
Chemotrops are further divided into two groups on the basis of source of electron Chemolithotrops they gain energy from oxidation of chemical compound and reduces inorganic compounds such as NH3 as electron source Eg NitrosomonasThe specific chemical exergy of water is (3110) b chw = g w(p 0, T 0) − μ w, 0 where g w(T 0, p 0) is the specific Gibbs potential of water at ambient pressure and temperature and μ w, 0 is the chemical potential of water in the RE If the chosen RE were the saturated air, then μ w, 0 = μ v(p s(T 0)), where p s is the saturationChemical energy is stored as food for us to use This energy came from the Sun Energy in our food is stored in chemical bonds Plants transform the sun's radiant energy into a "glue" for chemicals like sugar (sucrose) The plants store the sugars




Types Of Energy Knowledge Bank Solar Schools




A The Scaled Ground State Energy Eg Ef B The Chemical Potential Download Scientific Diagram
EWG's Skin Deep ® cosmetic database gives people practical solutions to protect themselves and their families from everyday exposures to potentially toxic chemicals in personal care and beauty products Skin Deep ®, launched in 04, lists easytonavigate hazard ratings for nearly 70,000 products and 9,000 ingredients on the marketThe US government doesn't review the toxicityChemical Engineering Graduation Requirements University of Washington https//chemewashingtonedu CHEM E 310 (4cr) Material and Energy Balances CHEM E 325 (4cr) Energy & Entropy CHEM E 326 (4cr) Chem Engineering Thermodynamics AMATH 301 (4cr) eg Scientific omputing (preferred) OR CSE 142 (4cr) Computer Programming IENERGY CHANGES IN CHEMICAL REACTIONS 1) Heat ( q ) is the transfer of energy from one substance to another An exothermic reaction is one in which energy is released eg C O (g ) O (g ) (g ) 93k J 2 1 2 →C O 2 3 With this type of reaction, H (products) < H (reactants)



1



Http Www Flippedoutscience Com Uploads 2 7 8 2 Energy Transformations Notes Pdf
A fuel cell is a device that converts the chemical energy from fuel into electricity via a chemical reaction with oxygen or another oxidizing agent Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols are sometimes used Fuel cells are different from batteries in that they require a constant source of fuel andWhen a chemical bond is broken, it is usually accompanied by a release of energy Similarly, the formation of chemical bonds requires an input of energy The energy supplied/released can be of various forms (such as heat, light, and electricity)> Nuclear Energy Nuclear energy is stored in the nucleus of atoms This energy is released when the nuclei are combined (fusion) or split




Chapter 3 Energy Work Work W




Readings Energy
Chemical potential energy is the energy stored in the chemical bonds of a substance The various chemicals that make up gasoline contain a large amount of chemical potential energy that is released when the gasoline is burned in a controlled way in the engine of the car The release of that energy does two thingsThe chemical exergy is an ideal mixture of ideal gas N as follows 1,3,4 (57) e ¯ M, i g C H = ∑ k = 1 N x k e ¯ k C H R ¯ T 0 ∑ k = 1 N x k L n x k If the temperature of the environment is T0, e ¯ k C H is the chemical standard molar mass of the k th chemical material, and xk is the mole fraction of k in the system at T0 1EG Chemistry Co, Ltd Company Description EG Chemistry leads new millenium in the fields of Excision Chemical Industry and Environmental Industry in the WorldWe provide complete corrosion protection in variety of forms to suit your steel products




Examples Of Chemical Energy Lovetoknow
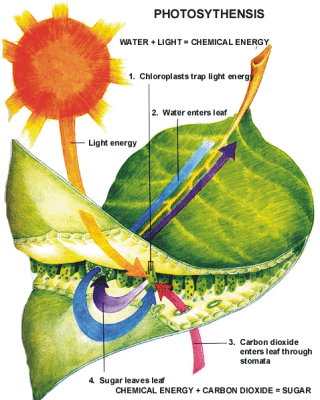



How Is Chemical Energy Used
260 Energy & EnvironmentVol 18, No 2, 07 "The concentration of CO 2 in the atmosphere has risen from close to 280 parts per million (ppm) in 1800, at first slowly and then progressively faster to a value of 367 ppm in 1999, echoing the increasing pace of global agricultural and industrialChemical energy definition at Dictionarycom, a free online dictionary with pronunciation, synonyms and translation Look it up now!The journal will invite review articles in all areas of chemistry, chemical engineering and materials science related to the design, production and use of materials for energy applications in a chemistry context, the priority areas include Sustainable utilization of raw materials Waste reduction, waste capture and recycling




Chemical Energy Kids Britannica Kids Homework Help




Chemical Energy Knowledge Bank Solar Schools
WELCOME FROM THE DEAN "On behalf of the School faculty, staff, and students, I would like to welcome you to the School of Energy Resources, Environmental, Chemical and Petrochemical Engineering (EECE) Our school has been providing innovative research in the fields of energy, environment, and chemical engineering since its foundation in 09 Currently, there are three departments The Department of EnergyChemical energy decreases as heat energy is released Endothermic reaction The system absorbs heat ( endo = going into ) from the environment due to weaker bonds being formed;It essentially adds enough chemical energy to allow the polymer to move around an reorder itself for several minutes before the polymer runs out of energy and sets More explanation here ABS plastic & Solvents 4 good ideas Sidenote Acetone can often instantaneously dissolve polymers with lots of styrene




Chemical Energy Knowledge Bank Solar Schools




Examples Of Chemical Energy Lovetoknow
Thermal energy is converted to chemical energyCL05) A chemical reaction has an activation energy of Eg= kcal/mol and a frequency factor of A=1012 1 What is the value of the rate constant k=A expEa/(RT)) at T=300 K? The sum of the initial stored energy, Eg in this case, is equal to the energy (s) in the final bar graph Therefore, the expression would read Eg W= Ek Eg Ediss
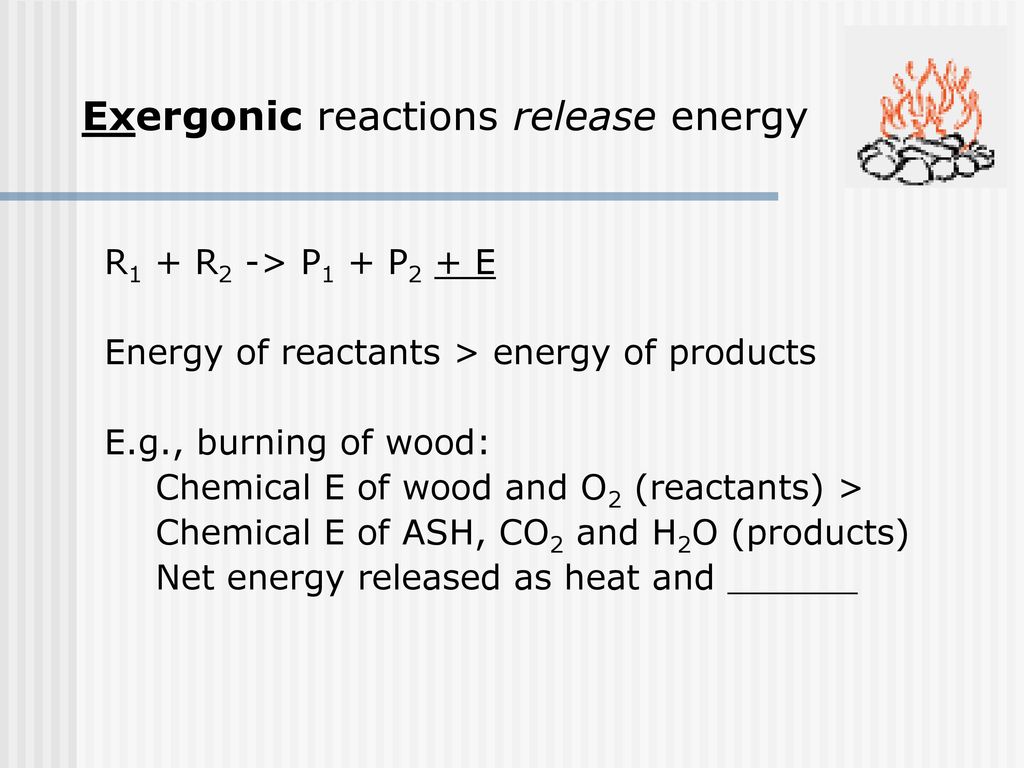



The Harvest And Storage Of Chemical Energy Ppt Download




Chemical Energy An Overview Sciencedirect Topics
The chemical energy in form of ATP is then used in biosynthesis to fix carbon in order to produce organic compounds * This process is different from photosynthesis where autotrophs are able to produce their own energy by using energy from the sun (sunlight) Because chemolithotrophs do not have access to sunlight, they have to rely onThe energy from macronutrients comes from their chemical bonds This chemical energy is converted into cellular energy that can be utilized to perform work, allowing cells to conduct their basic functions Although vitamins also have energy in their chemical bonds, our bodies do not make the enzymes to break these bonds and release this energySuch reactions are called exothermic Reactions that require an input of heat to proceed may store some of that energy as chemical energy in newly formed bonds The chemical energy in food is converted by the body into



Energy Energy Benchmark Sc B The Student Recognizes Various Forms Of Energy E G Heat Light And Electricity Also Assesses B 1 2 3 B 1 2 4 Ppt Download




What Is Chemical Energy Chemical Energy Physics Examples Of Chemical Energy Youtube
Chemical energy is energy that is stored in chemicals, such as sugar and gasoline As chemical energy is stored energy, it is a type of potential energy, which is energy stored in objects due to1Related applications of EG and PGbased products includes their use in refrigeration systems, heat transfer and water heating systems, building air conditioners, solar energy units, automatic sprinklers, snow melting equipment, deicing fluids for planes, waterbased paints, pharmaceutical products, and freeze drying apparatus (66, 67, 68, 69)Data R=14J/mol K) 1 cal=4184) 27 x 1012 s 0 27×1012, 1 1012 368 x
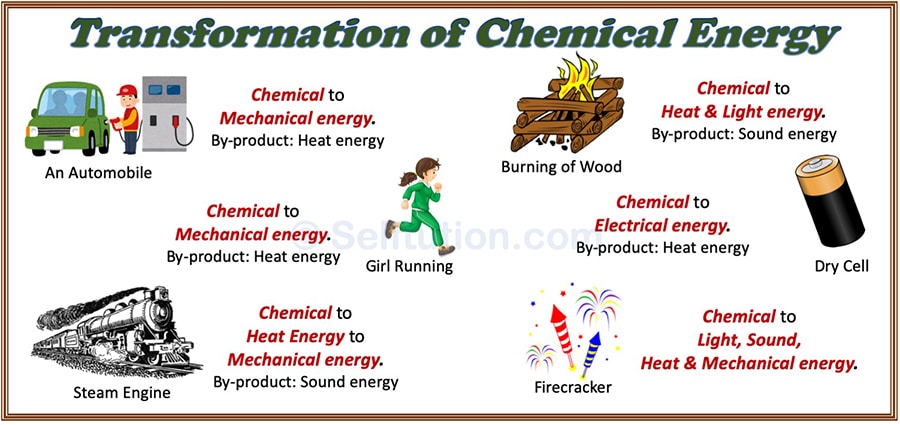



Transformation Or Conversion Of Energy With Examples Selftution



Ei Lehigh Edu Learners Energy Readings Energy Basics Pdf
Chemical energy is a form of potential energy that will only be observed when it is released in a chemical reaction Chemical energy is the energy of chemical bonds and is also stored in atoms and ions Interestingly, when chemical energy is released, the substance from which the energy came is often changed into an entirely new substanceAlso, high quality research contributions describing original unpublished results of conceptual, constructive, empirical, experimental, or theoretical work in all areas of Chemical, Energy and Environmental Engineering are invited to be presented at the conference Please visit https//abstracticceeeejustedueg and submit your abstract HOUSTON (ICIS)The parent companies of Gulf Coast Growth Ventures, a joint venture of ExxonMobil and SABIC, announced on Monday that mechanical completion of the polyethylene (PE) and ethylene glycol (EG) facilities at their new petrochemical complex near Corpus Christi, Texas, has been achieved




What Is Chemical Energy Definition And Examples



3
Chemical energy, Energy stored in the bonds of chemical compounds Chemical energy may be released during a chemical reaction, often in the form of heat; Those bacteria gain energy from chemical compounds;This lecture is about Chemical Energy and different examples of chemical energy It will teach you the calories of chemical energy and heat energy After wat




Chemical Energy
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



12 Examples Of Chemical Energy



Oxidative Phosphorylation




Chemical Energy An Overview Sciencedirect Topics
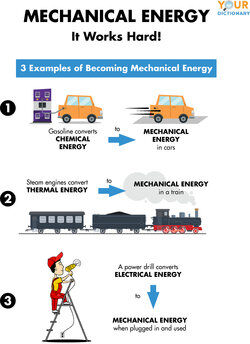



Examples Of Mechanical Energy At Home And In Daily Life




Energy Wikipedia



Mechanical Energy Wikipedia




What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com



Elasto Chemical Energy Of G Assuming That The Mechanically Stronger A Download Scientific Diagram



What Is Chemical Energy Definition Examples Video Lesson Transcript Study Com
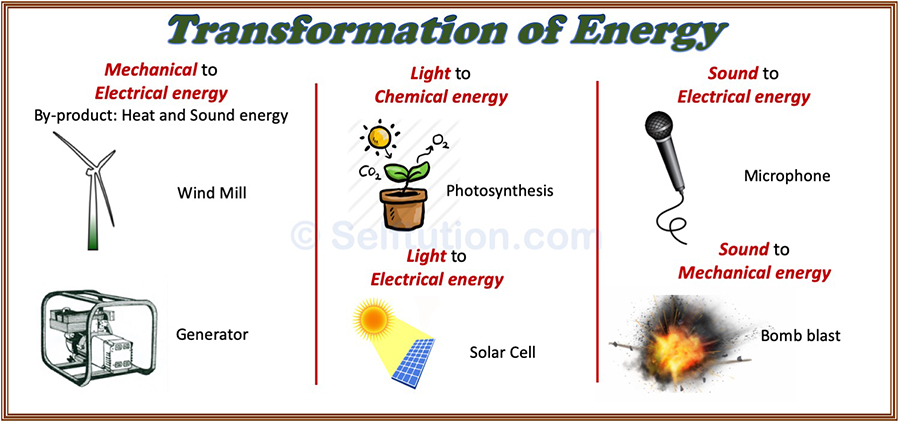



Transformation Or Conversion Of Energy With Examples Selftution




Types Of Energy Types Of Energy Electrical Energy Light Energy Sound Energy Thermal Energy Chemical Energy Nuclear Energy Mechanical Energy Each Can Ppt Download




Mechanical Energy Wikipedia




Biology Atp Flashcards Quizlet




Chemical Energy Kids Britannica Kids Homework Help




Themodynamics Metabolism Change Refers To All The Chemical Reactions That Change Or Transform Matter And Energy In Cells Metabolic Pathway A Sequential Ppt Download




Examples Of Chemical Energy Lovetoknow




Potential Energy Definition Examples Facts Britannica




Examples Of Chemical Energy Lovetoknow



Transformation Or Conversion Of Energy With Examples Selftution
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



12 Examples Of Chemical Energy




10 Examples Of Mechanical Energy In Everyday Life Studiousguy
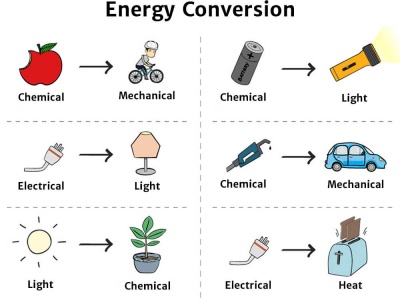



Energy Conversion Knowledge Bank Solar Schools
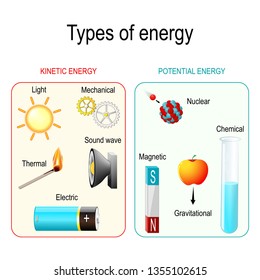



Kinetic Energy Hd Stock Images Shutterstock




Chemical Energy Energy Education
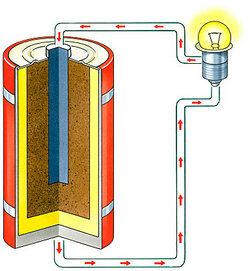



Examples Of Chemical Energy In Everyday Life




What Is Electrical Energy Definition Examples Video Lesson Transcript Study Com
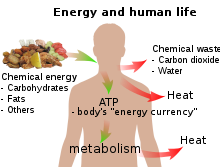



Energy Wikipedia



What Are The Uses Of Chemical Energy Quora




Online Chemical Awareness Course Environex International Pty Ltd




What Is Chemical Energy Definition And Examples
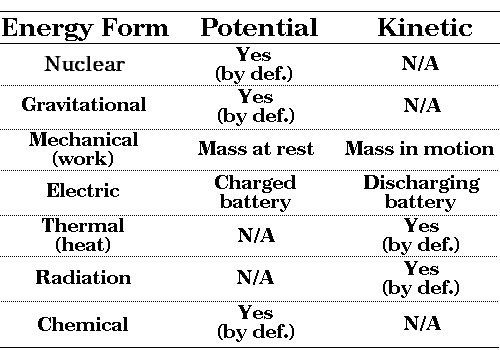



Matsc 101 Energy Fundamentals 3



Http Www Flippedoutscience Com Uploads 2 7 8 2 Energy Transformations Notes Pdf




Chapter 9 1 Energy Changes In Chemical Reactions Chemistry Libretexts




Chemical Energy Eschooltoday




Igcse Chemistry Energy Changes And Calorimetry Youtube




Photosynthesis A Metabolic Pathway That Converts Light Energy Into Chemical Energy Is The Process By Which Plants Some Bacteria And Some Protists Ppt Download
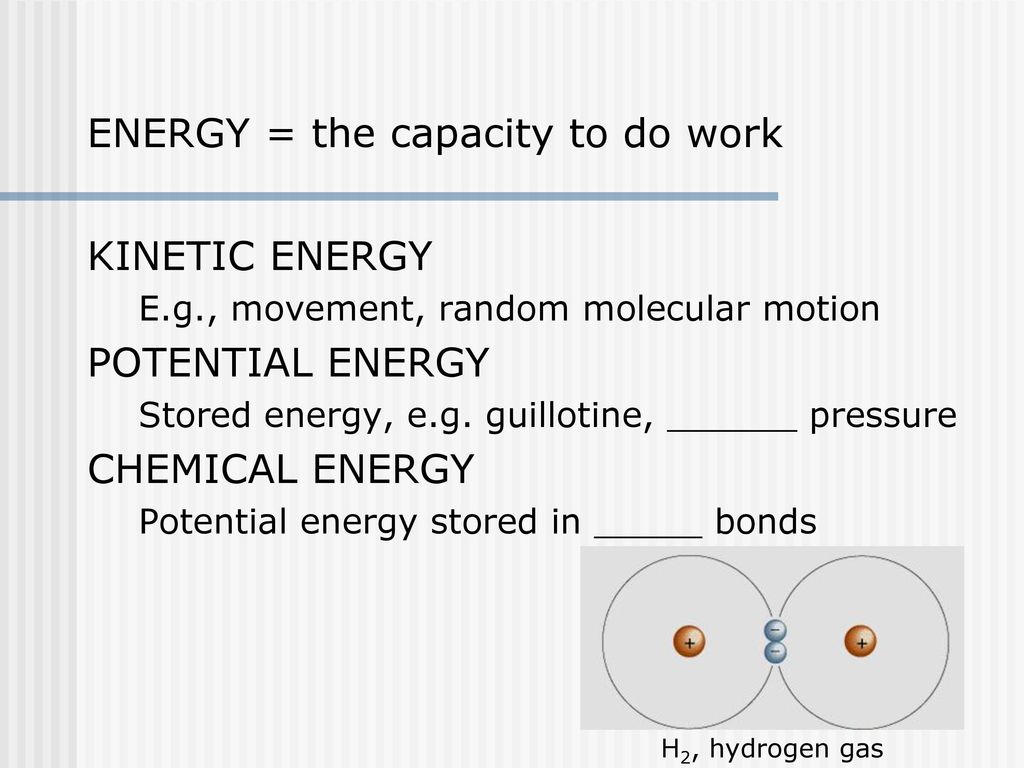



The Harvest And Storage Of Chemical Energy Ppt Download




Chemical Energy Knowledge Bank Solar Schools




What Is Kinetic Energy Kinetic Energy Examples



Chemical Energy Energy




Chemical Energy Definition Facts Example Eschool




Icceee




Energy Energy Equations Remember The Law Of Conservation Of Energy Well Energy Equations Show How This Energy Is Transformed E G Battery Chemical Potential Ppt Download




How Plants Transform Sunlight Into Food Biological Strategy Asknature
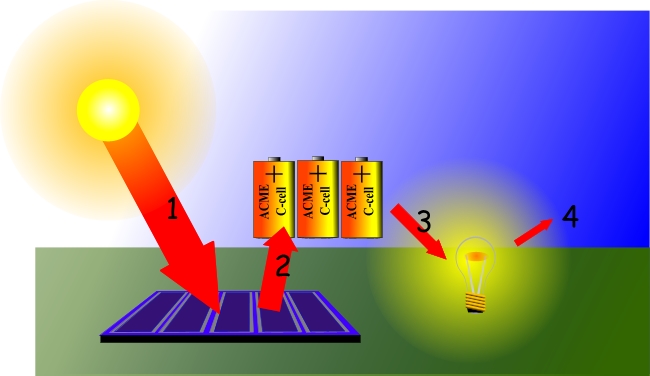



Energy From The Sun Green Plants As Primary Food Producers Tomatosphere First The Seed Foundation




What Is Chemical Energy




10 Types Of Energy And Examples




Chemical Reaction Wikipedia
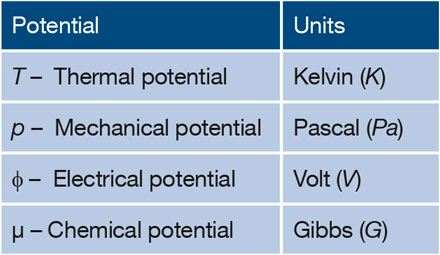



Chemical Potential And Gibbs Free Energy Mrs Bulletin Cambridge Core
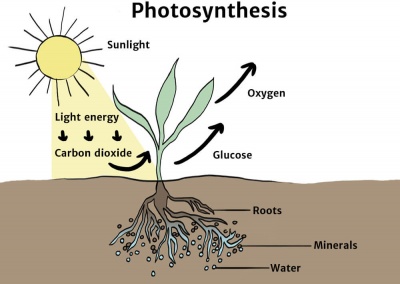



Chemical Energy Knowledge Bank Solar Schools



Mres Green Chemistry Energy And The Environment Study Imperial College London



The First Law Of Thermodynamics Introduction To Chemistry




Energy Changes In Chemical Reactions Introduction To Chemistry




What Kind Of Energy Does Gasoline Have Quora




Chemical Energy Storage




Work Power Energy Learning Outcomes Define Work Energy




Types Of Energy Article Khan Academy




Energy Transformation Definition Types Examples Video Lesson Transcript Study Com




Chemical Energy Kids Britannica Kids Homework Help
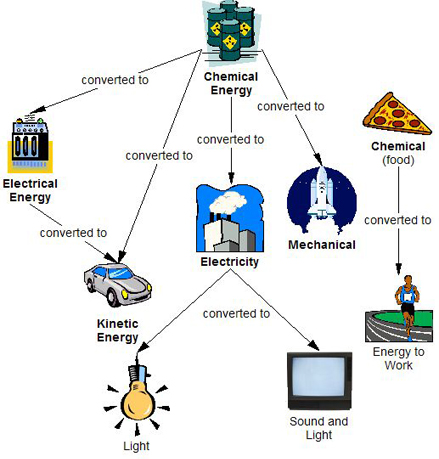



Energy Conversion Egee 102 Energy Conservation And Environmental Protection




Potential Energy Examples Examples Of Potential Energy Potential Energy Physics Youtube




What Is Energy Energy Definition And Examples Science



Q Tbn And9gctix7a3cwxjn Lxxcmuts15xob11nyfjdvwooc Nztl54prsu79 Usqp Cau




Energy Sources In An Eaf E G Electrical Energy And Chemical Energy Download Scientific Diagram




Different Types Of Energy With Everyday Examples




Chemistry And Light Www Scienceinschool Org




Potential Energy Definition Examples Facts Britannica
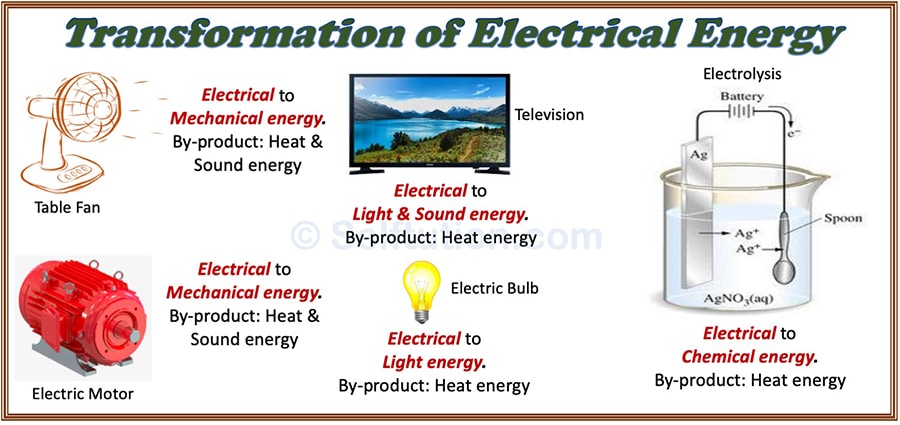



Transformation Or Conversion Of Energy With Examples Selftution



1
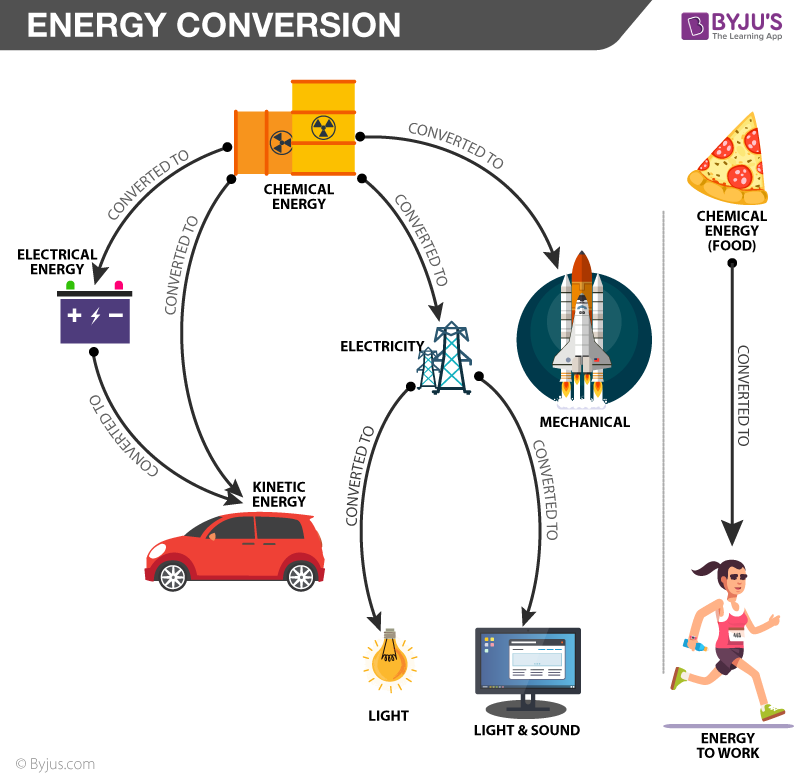



Energy Conversion Law Of Energy Conversion With Examples




Energy Forms Chemical Nuclear Electrical Heat Gravitational Light




Energy




Block3 Html



Chapter 14 Heat Work Energy Enthalpy 1 The
/close-up-shot-of-a-burning-piece-of-wood-94163322-5af443bac5542e0036a65348.jpg)



Chemical Energy Glossary Definition




Energy And Metabolism Boundless Biology
/example-of-chemical-energy-609260-final-bbb1d1f37ef443ad82bc2f2cdb2646ce.png)



12 Examples Of Chemical Energy




Chemical Energy Knowledge Bank Solar Schools
:max_bytes(150000):strip_icc()/biology_thermodynamics-584ef5785f9b58a8cd3da04e.jpg)



12 Examples Of Chemical Energy



No comments:
Post a Comment